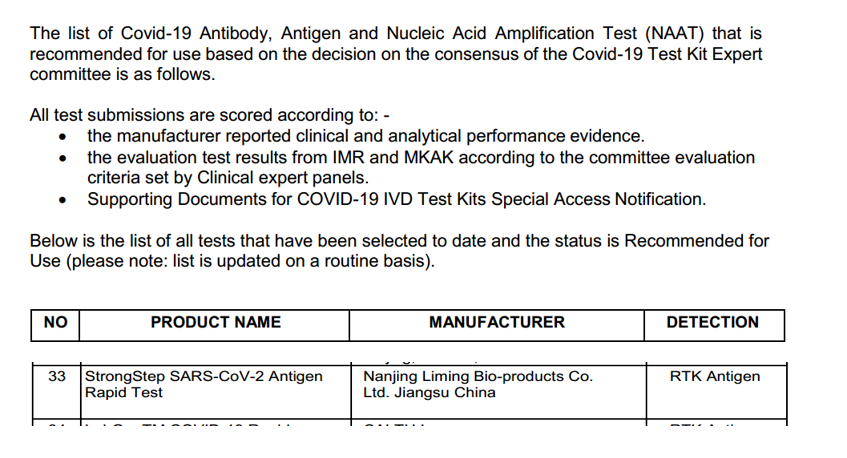
Posachedwapa, StrongStep® SARS-CoV-2 Antigen Rapid Test (Professional Edition) kuchokera ku Nanjing Liming Bio-products Co., Ltd. Human Services (DHSC) idawunikidwa paokha ndikuyamikiridwa.
Izi zisanachitike, zida zodziwira antigen za StrongStep® SARS-CoV-2 zochokera ku Nanjing Liming Bio-products Co., Ltd. Foundation inalimbikitsa mndandanda, chiphaso cha German Federal Agency for Medicines and Medical Devices (BfArM), , Guatemala certification, Indonesian FDA certification, Italy Ministry of Health certification, Philippines FDA certification, Singapore HSA certification, Ecuador certification, Brazil (ANVISA) certification, Chile certification , certification yaku Argentina, Dominica certification, Guatemala certification ndi ziphaso zina.Pakali pano, South Africa, India, WHO's EUL, FDA's EUA, European whitelist ndi ntchito zina zolembera ziphaso zili mkati.
Gwero lachithunzi: Adalangizidwa ndi Unduna wa Zaumoyo ku Malaysia

Chithunzi: Singapore HSA certification
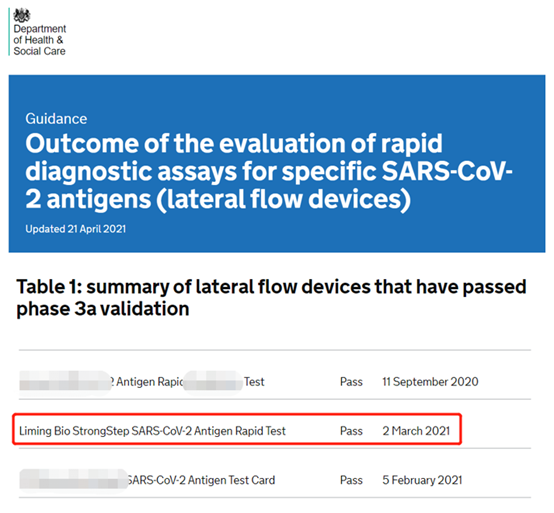
(Gwero la zithunzi: Tsamba lovomerezeka la DHSC la British Department of Health and Human Services)
Mu 2020, dipatimenti yoona za umoyo ndi ntchito za anthu ku United Kingdom idzatsimikizira kuti njira zodziwira matenda za COVID-19 zomwe zimalowa mdziko muno ndi zolondola komanso zodalirika.Pali zinthu 120 zomwe zikutenga nawo gawo pakutsimikizira, zomwe 19 zokha zadutsa kutsimikizira.Pambuyo pa miyezi 6 yotsimikizirika mobwereza bwereza, zitsanzo zabwino 200 ndi zitsanzo 1,000 zolakwika zinatsimikiziratu kugwira ntchito kwapamwamba kwa Nanjing Liming Bio-products Co., Ltd.
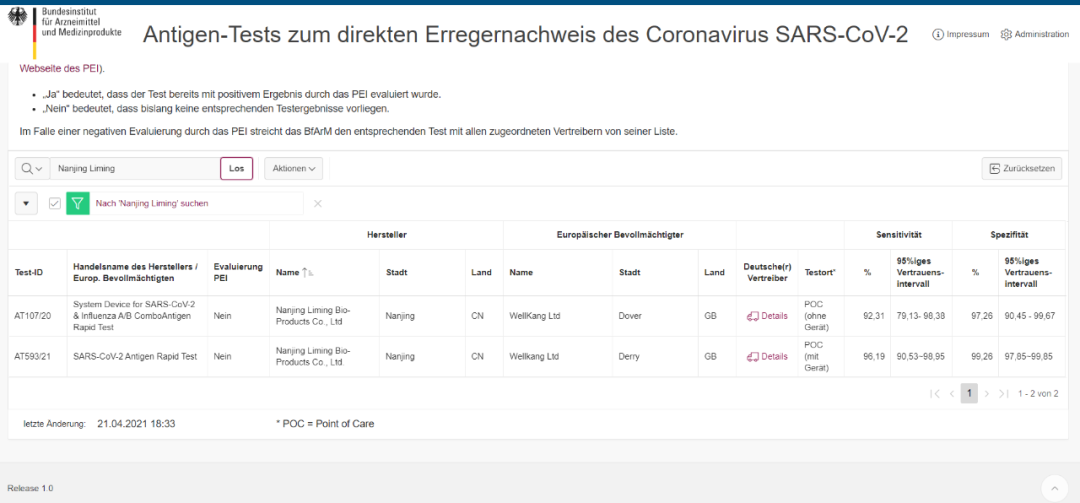
(Magwero a zithunzi: webusayiti ya Germany Federal Agency for Medicines and Medical Devices (BfArM))
Mayeso Ovomerezeka Ovomerezeka: AT593/21
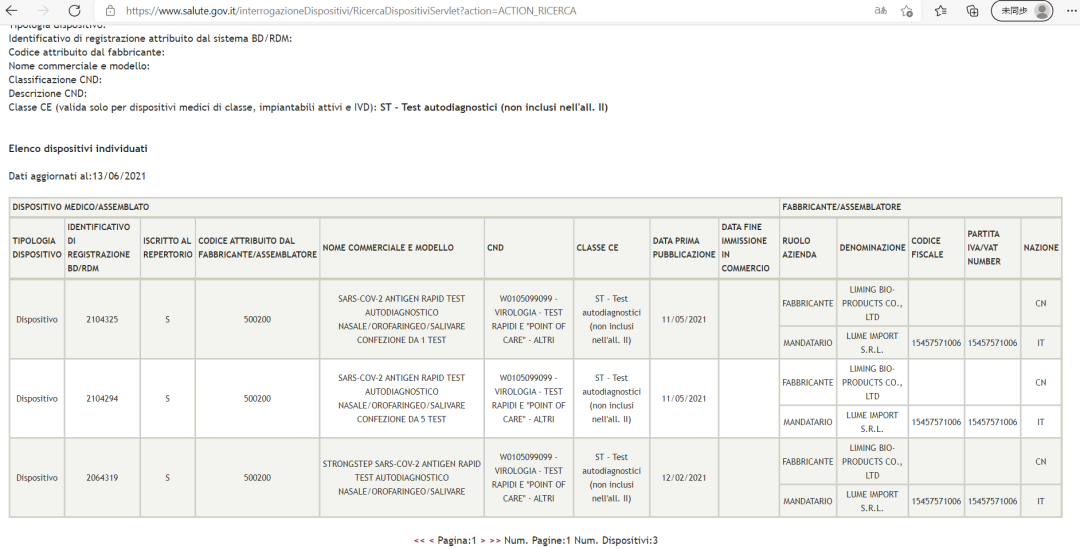
Mtundu wodziyesera wa StrongStep® SARS-CoV-2 Antigen Rapid Test (gulu lodziyesa) lavomerezedwa ndi Unduna wa Zaumoyo ku Italy.
Gwero: Tsamba lovomerezeka la Unduna wa Zaumoyo ku Italy (Ministero della Salute)

StrongStep® SARS-CoV-2 Antigen Rapid Test idayamikiridwa ndikuyamikiridwa ndi ogwiritsa ntchito aku Italy
Mayeso a antigen a SARS-CoV-2 ndiwofulumira, olondola, osavuta kugwiritsa ntchito, ndipo amafunikira zida zotsika komanso ogwira ntchito.Ndi bwino kwambiri kufufuza mofulumira milandu amaganiziridwa lalikulu latsopano koronavirus matenda, makamaka kwa mofulumira matenda a anaikira miliri.Itha kugwiritsidwa ntchito ngati njira yoyamba yodzitetezera pakuwongolera mliri, yogwiritsidwa ntchito pozindikira matenda oyamba, kuthandizira kupewa ndi kuwongolera mliri, ndikuwongolera kufalikira kwa kachilomboka.
COVID-19 ikhala mliri wanthawi yayitali mtsogolomo, ndipo kufunikira koyezetsa kudzakwera kwambiri.Pazinthu zosiyanasiyana zogwiritsira ntchito, Nanjing Liming Bio-products Co., Ltd. yapanga mitundu yosiyanasiyana ya ma reagents a SARS-CoV-2, "SARS-CoV-2 nucleic acid kuzindikira + SARS-CoV-2 antigen kuzindikira + SARS-CoV- 2 kuzindikira kwa antibody + SARS-CoV-2 / A ndi B antigen kuyesa katatu kofulumira + SARS-CoV-2 / A ndi B nucleic acid katatu kuyesa + SARS-CoV-2 kudziyang'anira banja "Yankho lathunthu limakwaniritsa zosowa kuzindikira ndi kupewa pamagulu onse pamsika wapadziko lonse lapansi.Thandizani mokwanira kupewa ndi kuwongolera mliri wapadziko lonse wa COVID-19 komanso kupewa ndi kuwongolera matenda opumira monga fuluwenza.
Nthawi yotumiza: Jun-23-2021







